MIDS Cardiac™
Cardiac troponin is well documented as the preferred biomarker for diagnosis of myocardial infarction due to its high sensitivity and specificity for myocardial injury. Evidence continues to show that high sensitivity troponin is emerging as one of the most powerful prognostic biomarkers for the assessment of cardiovascular risk in the general population. Over the past 10 years cardiac troponins T and I (cTnT and cTnI) have emerged as the cardiac biomarkers of choice for the diagnosis of Acute Myocardial Infarction (AMI, heart attack). High sensitivity troponin assays (hs-cTn) carried out on laboratory analyzers have yet to be approved for clinical use in the United States, although they are in clinical use in other parts of the world, including Europe, Canada, and Australia. There are no high sensitivity POC devices available for clinical practice in the “Golden Hour” which can compare with the growing use of these high sensitivity central laboratory cTnT and cTnI assays.
Laboratory analyzers and Point of Care tests are generally carried out by manipulating tagged magnetic assay beads and using optical technology to detect them. Large central laboratory analyzers use state of the art optical detection equipment during this process and have the capabilities to run confirmatory multiple tests simultaneously, and embody other techniques to achieve high sensitivity results. However, the major drawback is a slow turnaround time (TAT), when transport, testing and analysis times are taken into account. POC devices, due to their considerably smaller size, cannot effectively miniaturize the same technology. They are limited to optical detection of a lesser capability. In simple terms the assay beads are only viewed one or two dimensionally by the optical-sensing techniques and consequently only surface visible analytes are detectable. They are however regularly used because of their faster TAT, crucial when attempting to diagnose AMI, no other viable option being available when diagnosis time is so critical.
In contrast the MIDS technology is capable of detecting extremely low levels magnetic field disturbance (nano-Tesla) caused by the assay beads. This allows detection on a three dimensional level by detecting and measuring the aggregated magnetic signature of the assay beads using a lab-on-chip, multiplexed test strip.
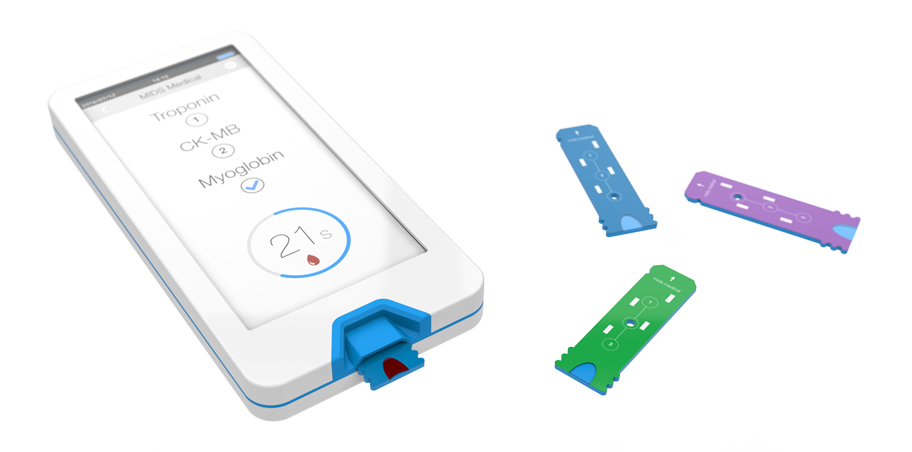
Incorporating the MIDS technology, MIDS Cardiac™is being developed to deliver the following key features and benefits:
- A step change in POC sensitivity and accuracy improvement, equal or superior to high sensitivity cardiac biomarker assays performed on central laboratory analyzers
- Patient friendly, finger prick sample rather than treated venous blood draw
- An industry leading tiny 5 microliter blood sample, 3 to 400 times smaller (1 assay) than existing POC devices for cardiac marker tests
- Test times 3 to 7 times faster (1 assay) than existing POC devices
- Capable of 3 multiplexed assays (3 cardiac biomarkers in a single test strip)
- Automated operation by minimally trained personnel even in an ambulatory setting
- Considerably more cost effective for healthcare providers than existing test devices
